Carbonates are the most common of the chemically precipitated minerals occurring in lacustrine sediment. In contrast with marine sediments, inorganically precipitated (though often biomediated) carbonates are widespread and sometimes dominant components of lacustrine sediment assemblages. When seen in smear slides, they are even more prominent than their abundance would suggest, due to their characteristically high birefringence when viewed by transmitted, cross-polarized light. In silt size ranges, no other common mineral component of lacustrine sediment displays the bright interference colors characteristic of the carbonate minerals. The mineral occurrence, form, size, and assemblages of associated constituents are all influenced by the chemistry and physical state of lake water from which they precipitate (as are other environmentally informative characteristics, such as isotopic composition and elemental substitution, not discernible by optical microscopy). You will also see carbonate minerals occurring as detrital sediment components, sometimes in abundance.
Calcite
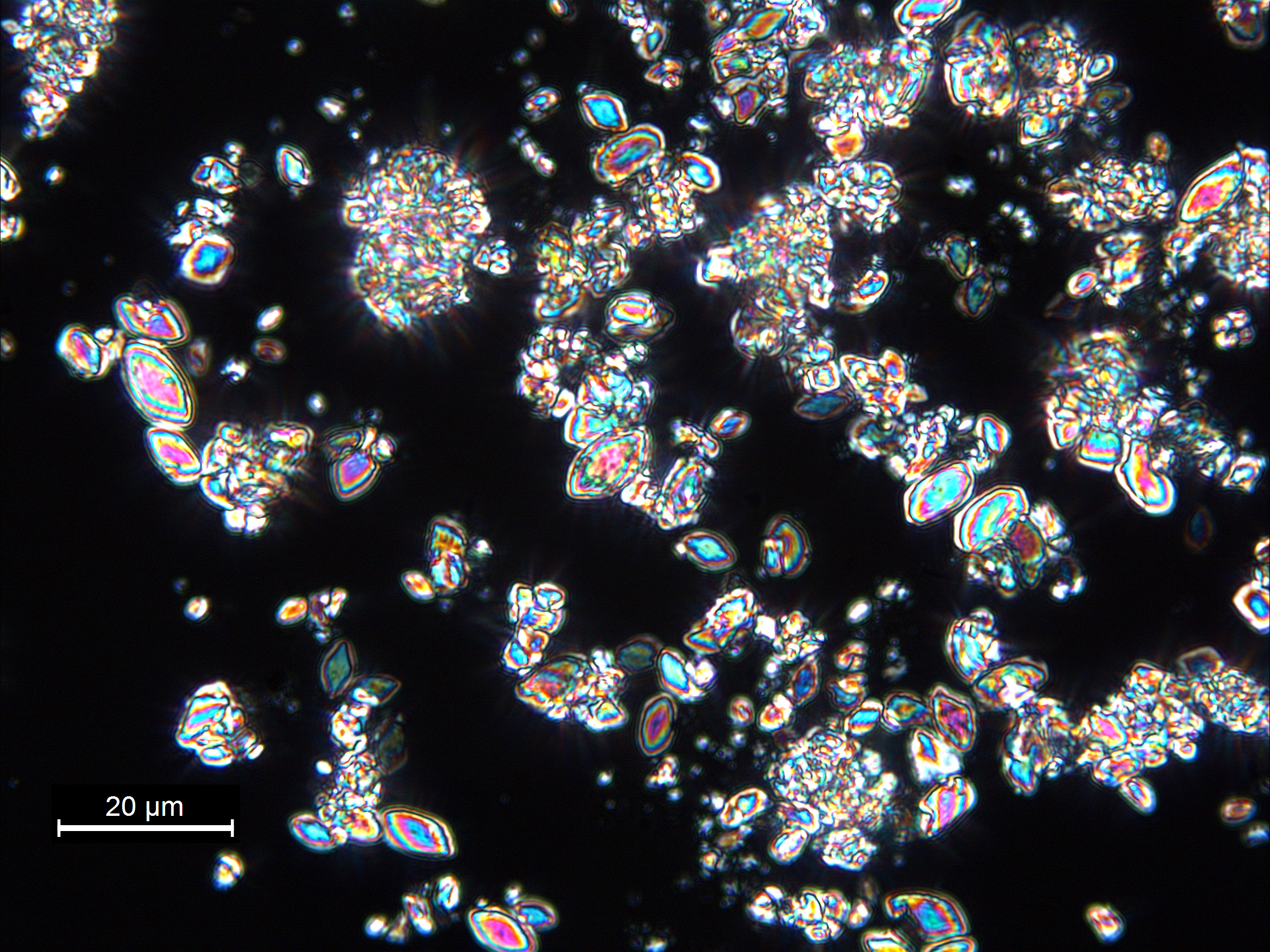
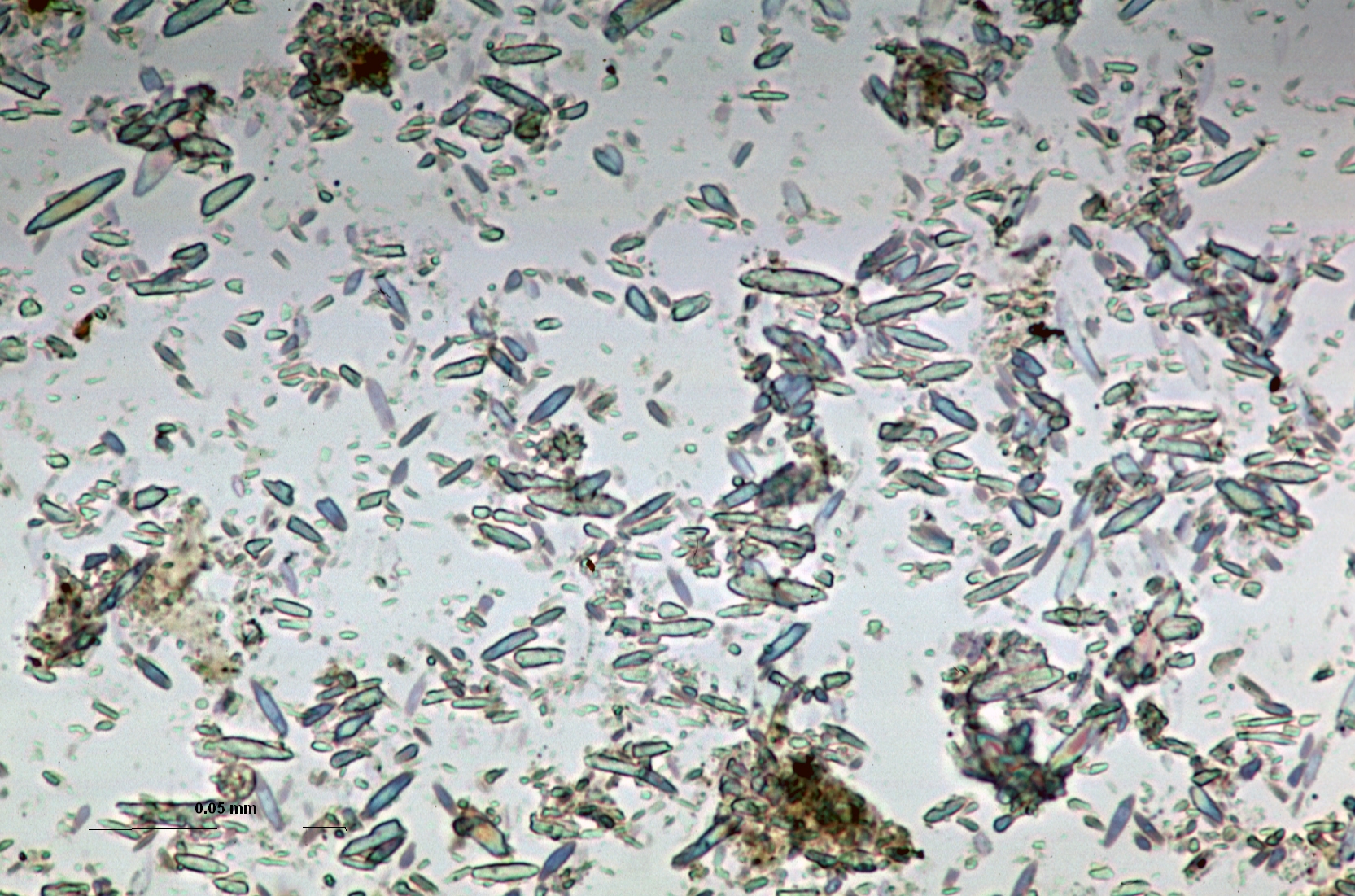
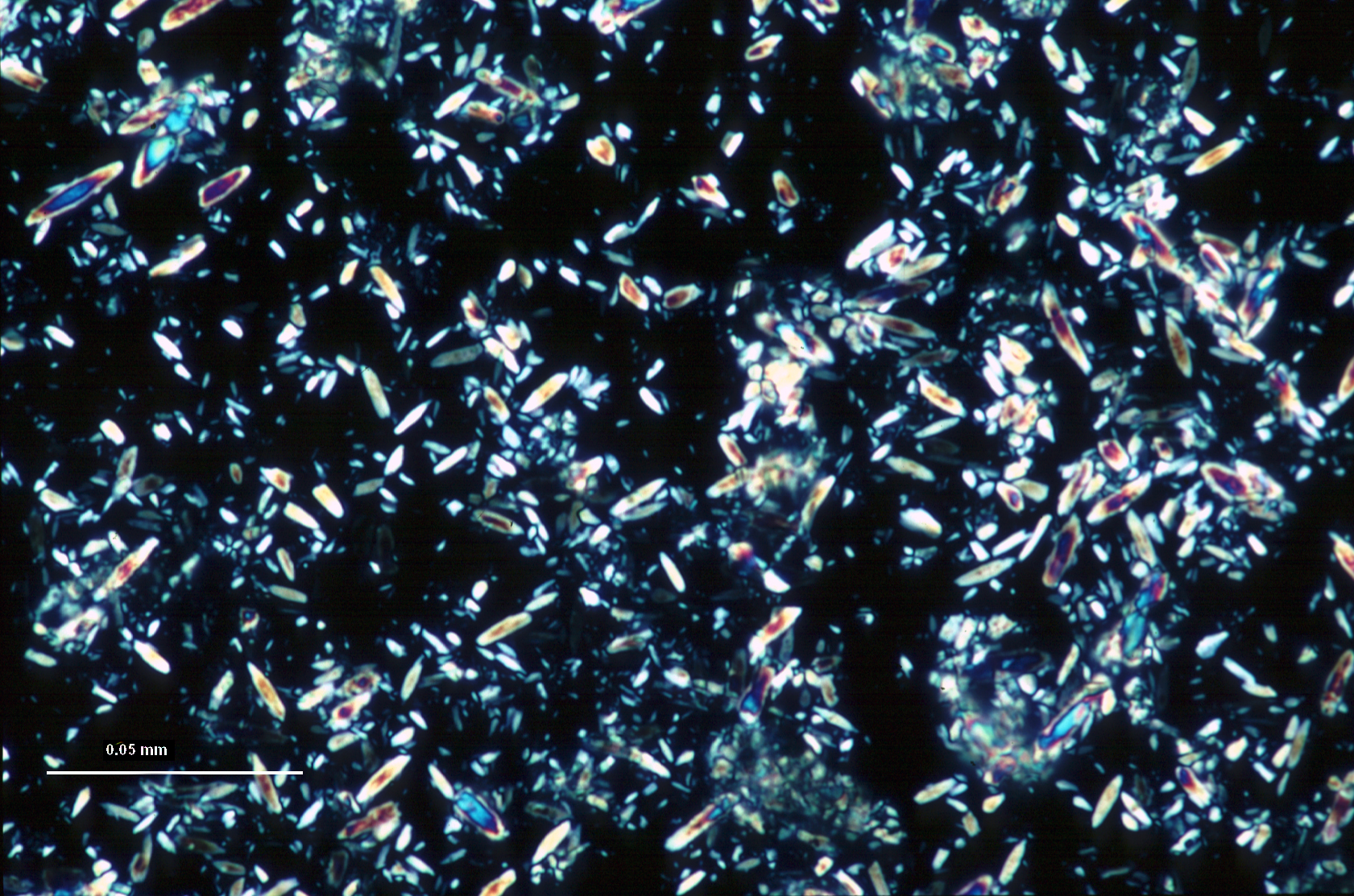
Calcite (calcium carbonate, CaCO3) is the predominant chemically precipitated carbonate mineral in most freshwater lakes. Lacustrine calcite forms as a primary precipitate in the water column of lakes, and also as a diagenetic mineral at or below the sediment-water interface. As a primary lacustrine precipitate (a process often biomediated through photosynthesis), calcite can take the form of regular rhombohedra following the crystallographic structure of the mineral, but often adopts forms that represent kinetically-controlled departures from the euhedral growth structure. These commonly include ellipsoids, ‘footballs’, and twinned forms in which two or more grains grow in interpenetrating configurations. Although lacustrine calcite forms over a wide range of sizes, kinetics of crystal growth often result in a strong mode in grain size distribution, giving the calcite component a ‘well-sorted’ character. A commonly observed upper limit in dimension, around 30 μm, relates to grain settling rates in the water column.

 |
| Corroded calcite with resorption accentuated along cleavage planes; plane-polarized (top) and cross-polarized (above) light. Tahltan Lake, British Columbia, Canada. |
Aragonite
Lacustrine calcium carbonate occurs as two distinct minerals (or polymorphs) of CaCO3 having very different crystal structure: calcite and aragonite. Calcite is the less soluble (more stable) mineral phase, but its formation is inhibited by elevated ion activities of Mg2+, SO42-, and possibly other ions. When calcite precipitation is inhibited in this manner, aragonite will form as a primary lacustrine mineral phase. While the mechanism is complex and thresholds inexactly defined, generally lacustrine aragonite formation is recognized as an indication of elevated Mg2+:Ca2+ in lake waters, typically as a result of CaCO3 formation and resulting solute evolution (Bischoff and Fyfe 1968; Eugster and Lawrence 1978; Kelts and Hsu 1978). Also, partitioning of carbon and oxygen isotopes between carbonate mineral, solute, and water depends on which of the two calcium carbonate polymorphs precipitates. Distinguishing CaCO3 mineralogy is therefore an important step toward interpreting the depositional environment of lacustrine sediment, or inferring, for example, the isotopic composition of water. You will need x-ray diffraction (xrd) to identify mineral phases with certainty, but smear slide analysis can provide you with valuable initial identification to be confirmed by xrd.
Aragonite shares the high birefringence and strong interference colors (relative to grain size) of calcite and other carbonate minerals, but when formed as an inorganic precipitate in natural waters, aragonite most often forms needles or elongated ellipsoids <15 μm in length and often compared to rice grains in form. While this describes a typical lacustrine aragonite occurrence, you may see overlap in grain form between some aragonite and ellipsoidal calcite. Due to sediment mixing and temperature effects, you may also see aragonite and calcite co-occurring in a single smear slide, even though the favored polymorph is largely controlled by lake-water chemistry.
Dolomite shares the extreme birefringence of other carbonate minerals, often shows rhombic habit in smear slides, and may be distinguished with difficulty from calcite of similar habit by higher relief and more equant habit. Unambiguous distinction from other carbonate minerals is again an xrd task. Often thought of as a secondary mineral replacing other carbonates, dolomite is known as a primary lacustrine precipitate in certain hypersaline, magnesian environments with favorable microbial ecology (Baker and Kastner 1981; Krause et al 2012), where you might expect to see it associated with other, less common Mg-carbonates such as magnesite and hydromagnesite. In the absence of hypersaline conditions, dolomite seen in smear slides is probably a detrital sediment component.
Siderite and rhodocrosite
Siderite (iron carbonate, FeCO3) and rhodocrosite (manganese carbonate, MnCO3) are minerals with widespread but generally less-abundant occurrence as lacustrine sediment components. Lacustrine siderite may be strongly colored in plane-polarized light, ranging from pale green to orange-brown. Grain forms include square-ended laths, ellipsoids, and ‘footballs’ or lemon-shaped euhedra. Interpenetrating twins are common, and if you are seeing grains in the <15 μm range with a ‘bow-tie’ or ‘dog-bone’ appearance, there is a good chance you have siderite present. Photographed at high resolution in a split core face, lamellae or nodules of siderite will sometimes appear a distinctive bronze color in a linescan image. Rhodocrosite is greenish-brown in plane polarized light, may be strongly pleiochroic, and can display grain forms similar to siderite, including interpenetrating twins; distinguishing the two minerals reliably is a task for xrd analysis.
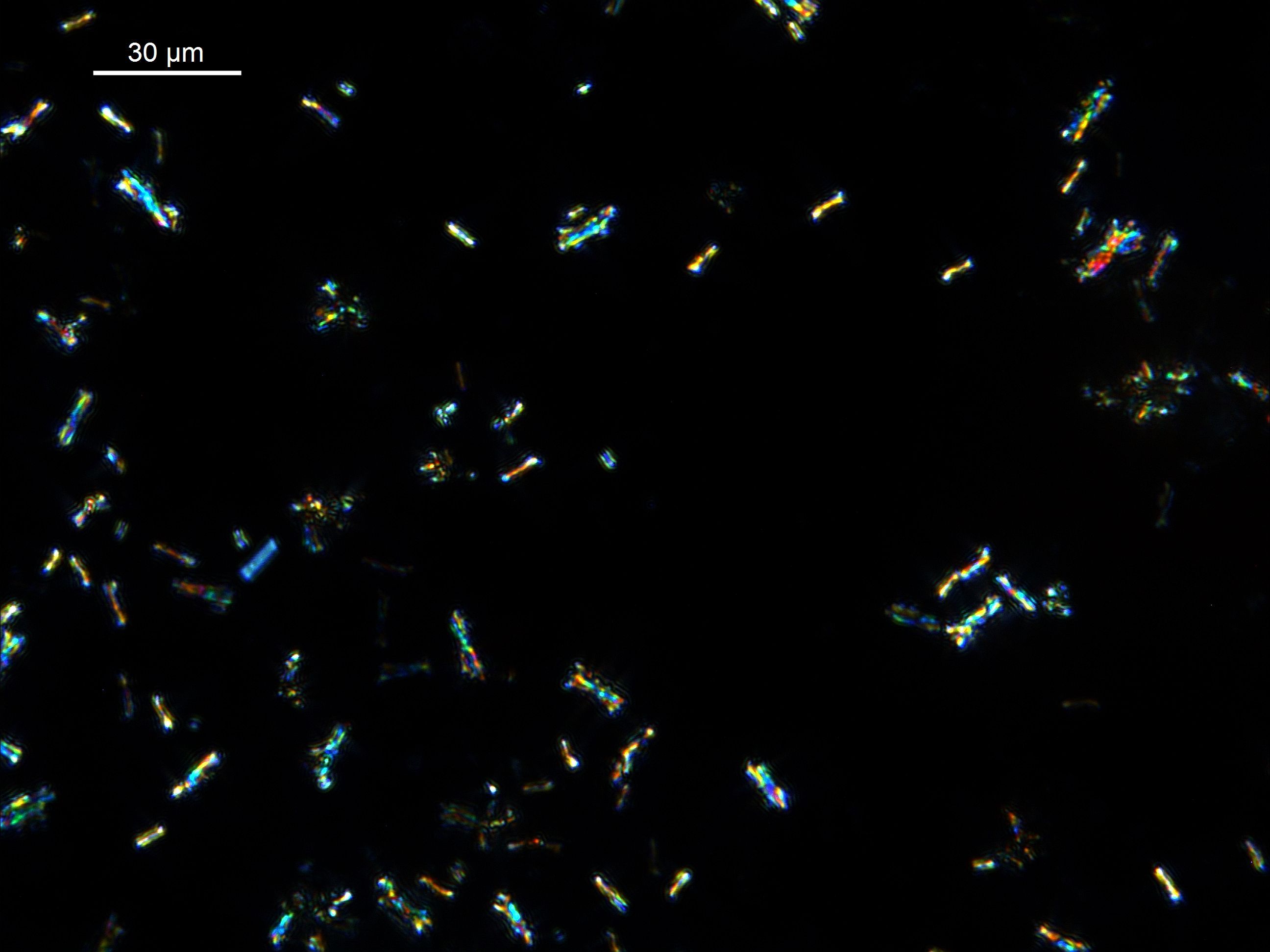 |
| Siderite in plane-polarized (top) and cross-polarized light. Grain forms include simple laths and twinned laths giving a 'bow-tie' form, accentuated under cross-polarized light. |
Overgrowths and secondary precipitation
Through the processes of settling through the water column, changes in water column stratification, and burial below the sediment-water interface, carbonate minerals may pass from saturated to unsaturated conditions and back again over the course of sediment accumulation. This can at times result in multiphase overgrowths, an indicator of oscillatory saturation state that may support useful environmental interpretations. Look for secondary rims on carbonate grains, which may or may not be in crystallographic unity with the host grain (indicated by coinciding or disjunct optical extinction of the host grain and its overgrowths as you rotate the microscope stage). In some interesting cases, a host carbonate grain may be overgrown by a different carbonate mineral reflecting equilibration in distinct parts of the lake water column (e.g., Stevens et al 2000).
References cited
Bade, D. L., Carpenter, S. R., Cole, J. J., Hanson, P. C., and Hesslein, R. H., 2004, Controls of d13C DIC in lakes: Geochemistry, lake metabolism and morphometry: Limnology and Oceanography, v. 49, no. 4, p. 1160-1172.
Baker, P. A., and Kastner, M., 1981, Constraints on the formation of sedimentary dolomite: Science, v. 213, p. 214-216.
Bischoff, J. L., and Fyfe, W. S., 1968, Catalysis, inhibition, and the calcite-aragonite problem: American Journal of Science, v. 266, p. 65-79.
Eugster, H. P., and Hardie, L. A., 1978, Chapter 8: Saline Lakes, in Lerman, A., ed., Physics and Chemistry of Lakes: New York, Springer-Verlag, p. 237-293.
Hem, J. D., 1977, Reactions of metal ions at surfaces of hydrous iron oxide: Geochimica et Cosmochimica Acta, v. 41, p. 527-538.
Kelts, K., and Hsu, K. J., 1978, Freshwater carbonate sedimentation, in Lerman, A., ed., Lakes: Chemistry, Geology, Physics: New York, Springer-Verlag.
Krause, S., Liebetrau, V., Gorb, S., Sanchez-Roman, M., McKenzie, J. A., and Treude, T., 2012, Microbial nucleation of Mg-rich dolomite in exopolymeric substances under anoxic modern seawater salinity: New insight into an old enigma: Geology, v. 40, no. 7, p. 587-590.
Lemos, V. P., Lima da Costa, M., Lemos, R. L., and Gomes de Faria, M. S., 2007, Vivianite and siderite in lateritic iron crust: and example of bioreduction: Quimica Nova, v. 30, no. 1, p. 36-40.
Myrbo, A., and Shapley, M. D., 2006, Seasonal water-column dynamics of dissolved inorganic carbon stable isotopic compositions (d13CDIC) in small hardwater lakes in Minnesota and Montana: Geochimica et Cosmochimica Acta, v. 70, p. 2699-2714.
Stevens, L. R., Ito, E., and Olson, D. E. L., 2000, Relationship of Mn-carbonates in varved lake sediments to catchment vegetation in Big Watab Lake, MN, USA: Journal of Paleolimnology, v. 24, p. 199-211.




No comments:
Post a Comment