After preparing your smear slides, view them using a petrographic (polarizing light) microscope. It is nice to have an external light source (such as a fiberoptic or LED illuminator) nearby to apply incident light to see the true color of opaque grains.
Scan around the whole slide at ~100x (typically the 10x
objective) to see whether sediment is distributed evenly. (For instance, larger grains sometimes go to
the edges of the wet area.) Find a
representative area or two to zoom in on.
Switch to 400x. Focus up and down to see particles in
different focal planes. Throughout the analysis, be sure to switch regularly between plane-polarized and cross-polarized light, because some components are difficult or impossible to see in one or the other.
Identifying components in smear slides
Contaminants. Some components are not meant to be in your slide. These rogue materials include bubbles in the optical cement, fibers from your clothes, and small particles of the toothpicks used to make slides. In slides prepared during more permissive times, you may find cigarette ash, which reportedly looks like a zeolite (Kelts 2003).
Identifying components in smear slides
Contaminants. Some components are not meant to be in your slide. These rogue materials include bubbles in the optical cement, fibers from your clothes, and small particles of the toothpicks used to make slides. In slides prepared during more permissive times, you may find cigarette ash, which reportedly looks like a zeolite (Kelts 2003).
The prominent circular objects you see, with very
high relief (dark edges) are bubbles in
the optical cement. You may also see
bubbles filling some microfossils – the heavy dark edges are the clue.
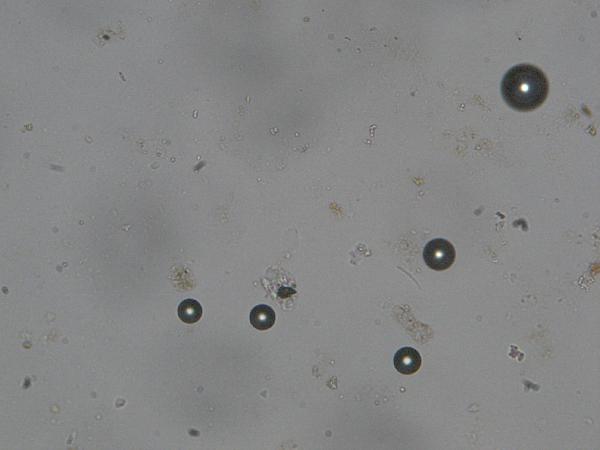 |
| bubbles |
 |
| bubbles within diatom frustules |
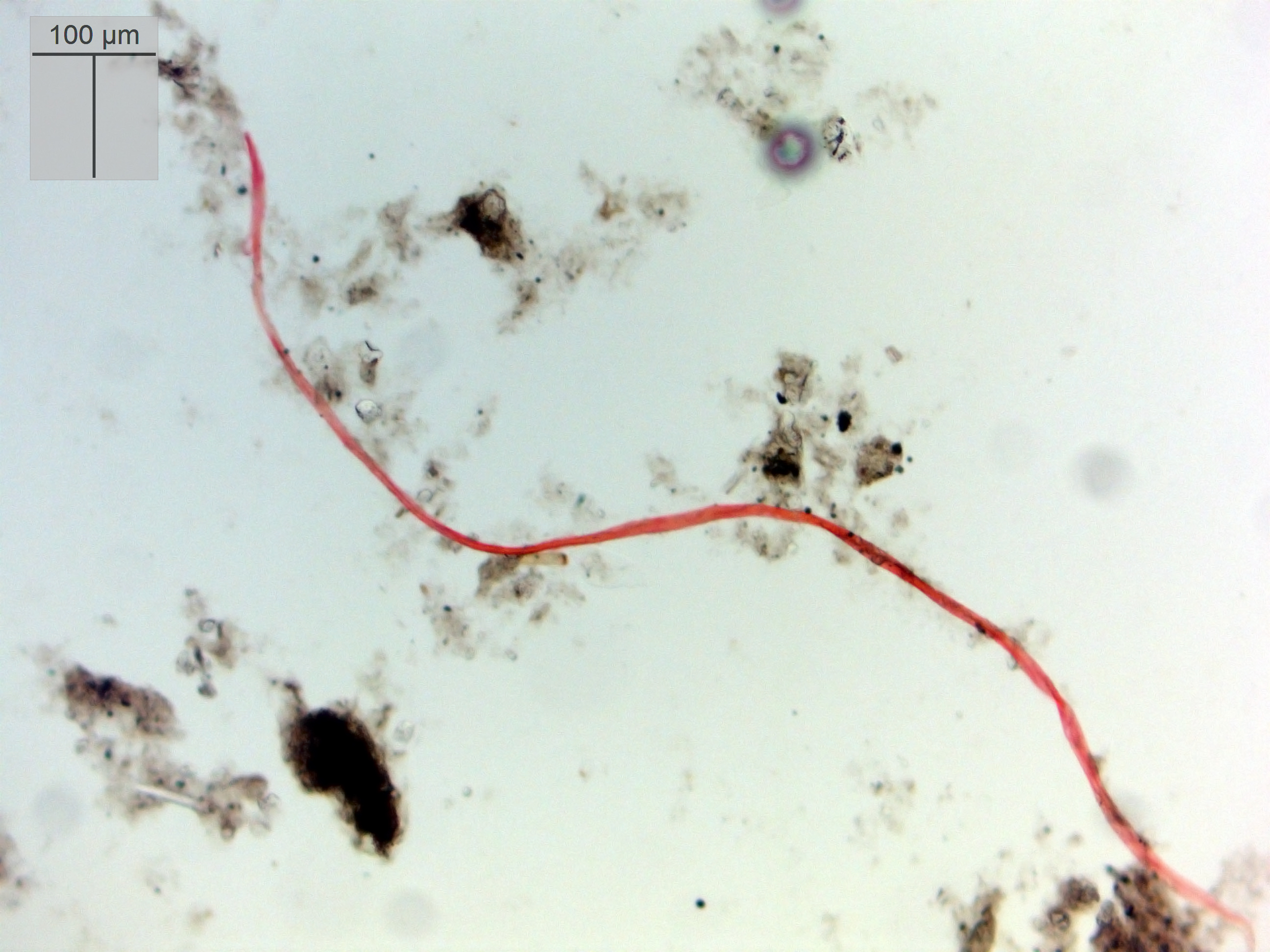 |
| a fiber |
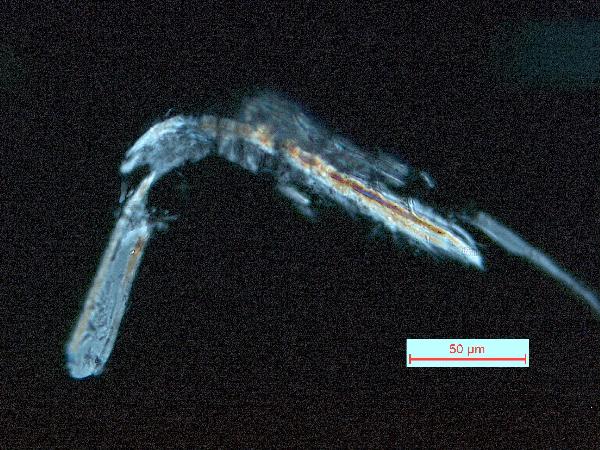 |
| toothpick shard in plane- and cross-polarized light |
The good stuff. In spite of the apparently great variety of materials in a smear slide, 99% of the particles you see belong to one of four groups. They are:
Organic matter
Diatoms
Siliciclastics
Carbonates
Let’s go through these one by one.
Organic matter
The translucent brown material you see is organic
matter – dead tissue. There is (1) the
amorphous brown goo with no structure, which is decomposing algal organic
matter, and there is (2) the brown stuff in which you can see cell
structure, which is the remains of "higher" (vascular) plants that
grew rooted on shore or in the water. This material is also termed "cuticle."
(There may also be remains of aquatic mosses, which are not vascular but
are not amorphous.) Both may be mixed or
encrusted with other subfossil remains, minerals, etc., but their identifying
characteristic is the translucent brown color (but see also insect remains and
chitin, below).
 |
| amorphous algal organic matter |
 |
| higher plant material showing cell structure |
There may also be (3) opaque, or nearly opaque,
brown to black particles of irregular shapes.
These are soil organic matter (also called carbonized organic
matter). If the particles are really
black (and if incident light shows them to be iridescent), they are charcoal. Charcoal may have more delicate structure
than soil organic matter because it is deposited via air rather than being
washed into the lake. Soil organic
matter will usually be associated with other terrestrial materials such as phytoliths
and clastic minerals, and may be subangular to subrounded in outline.
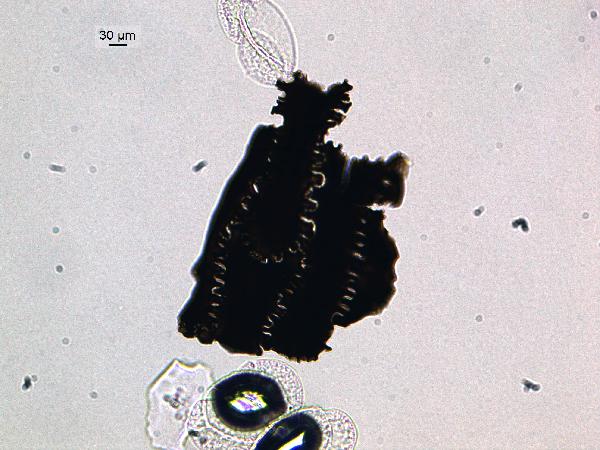 |
| charcoal |
Observations
to make. You usually can’t identify
the plant of origin from material in a smear slide. What you should do instead is to note the
relative abundances of amorphous, vascular, and soil organic matter. This provides environmental information - for instance, about productivity in the lake vs. material eroded in; or water depth/clarity.
Diatoms
The most eye-catching components of lake sediments
are diatoms. They come in many shapes, but are generally
cylindrical (appearing circular or rectangular depending on how they lie on the
slide) or elongate. The cylindrical ones
are radially symmetrical, are usually planktonic, and are called centric diatoms. The elongate ones are bilaterally
symmetrical, are usually benthic, and are called pennate diatoms. These
groups are not hard and fast, but it’s a good start. Diatom remains (frustules, the cell walls) are made of amorphous silica (like opal
or glass) and disappear under cross-polarized light because they are isotropic.
 |
| an assemblage of freshwater diatoms including planktonic and benthic forms |
Observations
to make. Identifying diatoms to
genus or species is an expert’s job, and one that can be difficult with a
petrographic microscope, at 400x, and with a sample mounted in optical cement. But even the
non-expert can gather some information by observing whether the diatom flora is
diverse or only has a few species; whether the diatoms are well preserved, or
broken or dissolved; and making some sketches of shapes to compare between
slides. (The simplest type of diatom
analysis is to count planktonic vs benthic or centric vs. pennate, which can
provide information on water depth and clarity.) All of these observations can provide
environmental information, and will also help you determine whether to bring in
a professional diatomist. Much more on diatom identification, taxonomy, and environmental significance can be found at Diatoms of the US.
Siliciclastics
Cross the nicols and observe the grains that light
up. Rotate the stage to make sure that
you are seeing all of them. Most of are
probably gray or white, and some large grains may have dark pink and orange
colors. There are also grains with green or brown body color that may mask
their birefringence colors. (For now,
ignore the grains that light up brightly in pastel colors. These are carbonates
and are part of the last group, below.)
The
gray and white grains are quartz and feldspar. Silt-sized grains of these minerals look similar to one another, but if a grain displays a straight edge, it's feldspar (since quartz has conchoidal fracture, and no cleavage).
 |
| quartz in plane- and cross-polarized light |
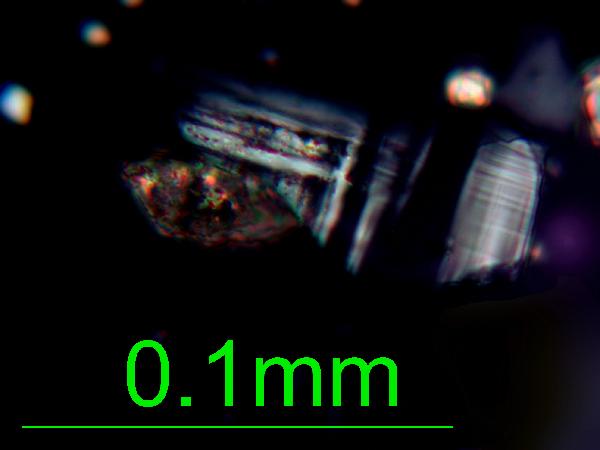 |
| feldspar (microcline) in plane- and cross-polarized light |
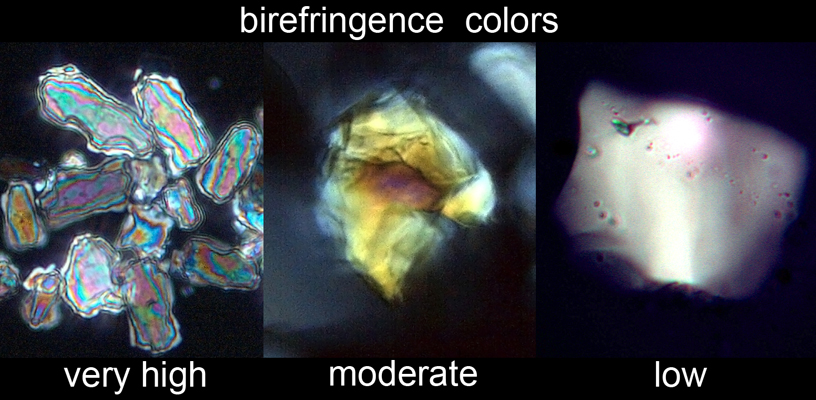
If you are accustomed to looking at thin sections, the birefringence colors of larger quartz grains (>40 μm) may confuse you: at these thicknesses, quartz is at the upper rather than the lower end of first-order colors - red, dark yellow, dark purple, etc. Contrast these colors with the pastel high-order birefringence colors of carbonates and the vivid medium-order birefringence colors of many heavy minerals.
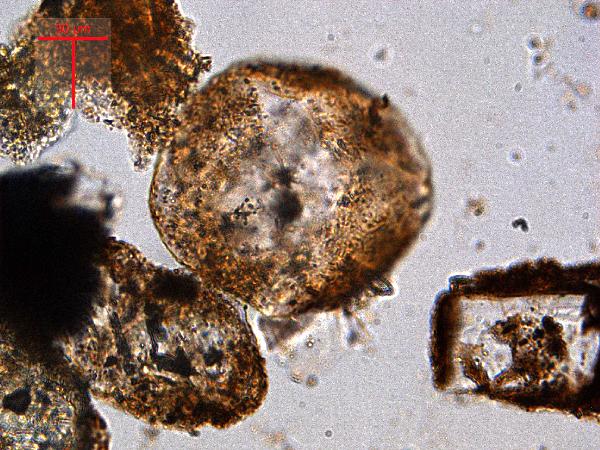 | ||
|
The green or brown (sometimes red)
grains are mafic (or ferromagnesian) minerals. The author's favorite in smear slide is olivine.
 |
| olivine (central high-relief grain) in plane- and cross-polarized light |
There are also heavy minerals
(e.g., epidote, tourmaline, zircon, garnet, rutile, apatite; and opaques such as magnetite, pyrite, marcasite), many of which have a
characteristic vivid medium-order birefringence and high relief.
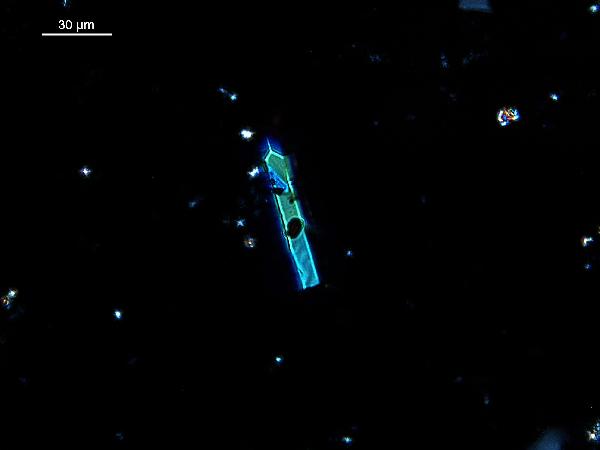 |
| tourmaline in plane- and cross-polarized light |
 |
| rutile (high relief twinned crystal) in plane- and cross polarized light |
It is not always necessary to identify every
silicate clastic grain in lake sediments.
Small lakes in uniform surficial geology will not usually vary over time
in their mineralogy (but see below for important textural observations that
should be made). In contrast, in large
lakes with, e.g., inlets draining different geologies, significant changes in mineralogy,
and thus provenance, can indicate climatic, tectonic, and vegetational
changes. For a primer on identification
of silicate minerals in smear slides, see the siliciclastics tutorial.
Observations
to make. Siliciclastic mineralogy is
important, but in small lakes perhaps not as important as noting the grain
size, sorting, and rounding of the silicate grains as a population. If your microscope is not calibrated so that
you can determine the actual size of objects in the field of view, it’s easy to
do so if you have an eyepiece micrometer (reticule) and a stage micrometer or
even a transparent ruler.
Get used to the size ranges of clay (below 4 μm or 2 μm, depending on whom you ask),
sand (>:63 μm),
and especially silt (4-63 μm, by far the most common grain size in lakes except
large lakes). It is easy to make a
semi-quantitative observation such as “clayey silt” or “silty sand” – which is
really all you need in order to make a first-order environmental
interpretation.
Carbonates
Calcite is
overwhelmingly the most common carbonate mineral in lake sediments, and indeed
the most common authigenic component of lake sediments. In contrast to the marine system, almost all
lacustrine carbonate is inorganically precipitated (though bio-mediated by the
changes in pH and carbonate saturation induced by photosynthesis) in lake surface waters. This authigenic calcite typically appears as regular
to corroded rhombs. (Dissolution or
corrosion occurs in lake bottom waters at low pH and low carbonate
saturation.) For more detail on identification and analysis of carbonates, see the carbonate tutorial.
 |
| calcite in plane- and cross-polarized light |
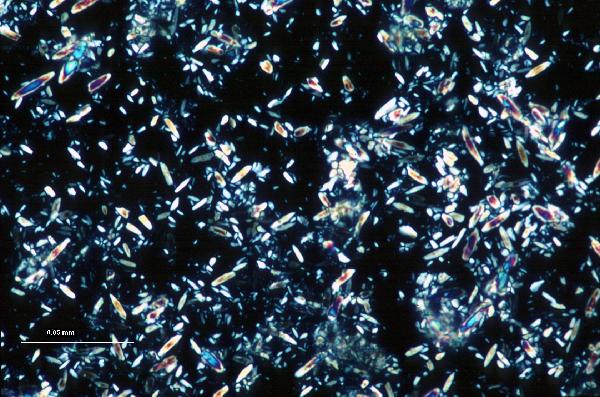 |
| aragonite in plane- and cross-polarized light |
Dolomite,
while rare, appears as tight, equant rhombs but overlaps with calcite in
appearance and the two phases can be readily confused.
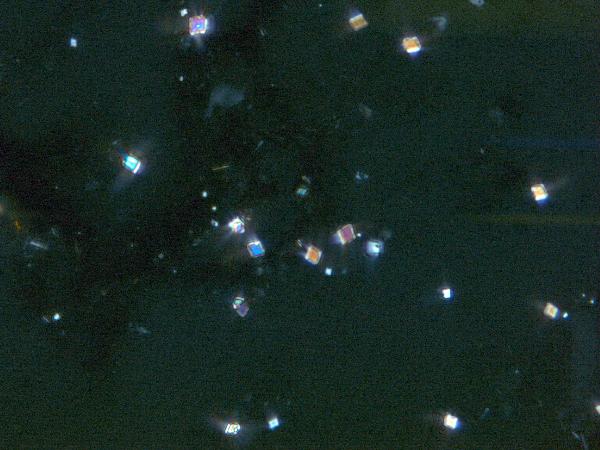 |
| dolomite in plane- and cross-polarized light |
The myriad iron-manganese-etc carbonates (such
as the relatively common siderite
and rarer rhodocrosite)
are frequently diagenetic (formed post-depositionally) or precipitated in lake
bottom waters (because they require reduced Fe and Mn species). They have been understudied, and are poorly
characterized environmentally, despite their exceedingly important role as
indicators of anoxia, alkalinity, and overturn.
The main distinguishing feature of carbonate
minerals is their high-order birefringence colors.
This is manifested best in large calcite grains as concentric rings of
pastel blues and pinks, but as a whole, carbonates look fundamentally different
from any silicate mineral. Thick grains
of quartz may show reds and oranges, but these are readily distinguished from
the diagnostic colors of carbonates.
We separate carbonates from other minerals because
they are a common precipitate in lakes – not just a detrital component – and so
they have their own environmental significance.
Detrital carbonates (derived from limestone bedrock or till) may be
present; they are typically >50 μm (authigenic carbonates can only grow to about 30 μm before they sink), polycrystalline, and beat up.
 |
| detrital calcite grain in plane- and cross-polarized light |
The calcifying green alga Phacotus has a calcite lorica, a spherical shell that falls apart in halves when the organism dies. It is a common component of the sediments in temperate hardwater lakes. It has a characteristic birefringence pattern that looks like baseball seams, or a pinwheel that turns as you rotate the stage. If you look closely, there are two distinct forms, which probably represent lorica halves lying concave-up and concave-down.
 |
| Phacotus in plane- and cross-polarized light |
Observations
to make. Carbonate mineralogy is a
good indicator of lake water chemistry and ionic strength, but you have to be
at fairly high ionic strength (and high Mg:Ca) to get anything but
calcite. If you only have calcite, there
are still important observations: How
large are the grains? Are they
authigenic or detrital? If both are
present, what is the size break between them (e.g., if you wanted to sieve out
the detrital calcite, what size mesh would you use?)? Do grains look nice, even, perhaps
rhombohedral (as the first example above), or are they scraggly and corroded? Do they look polycrystaline and chunky, as is characteristic of shallow-water carbonates (which may be associated with plant matter)?
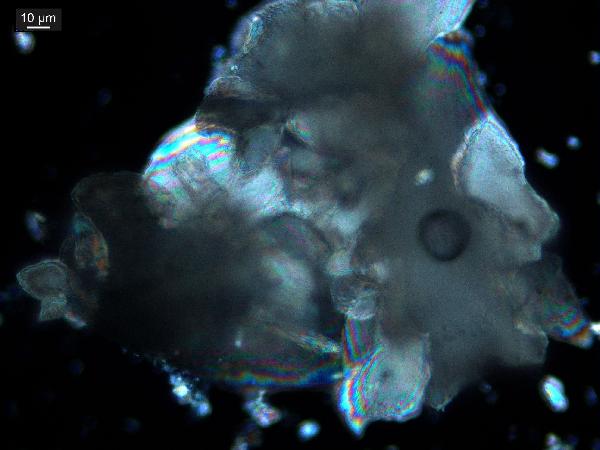 |
| characteristic shallow-water polycrystalline calcite grain in plane- and cross-polarized light |
Other components
Less abundant but environmentally significant sedimentary grains include volcanic glass and diagenetic and biological components.
Volcanic glass is easy to identify in smear slides because it is isotropic. Glass shards may also have distinctive morphological features such as bubbles or broken bubble walls, stretched textures, and scythe-like edges, but isotropy is the diagnostic feature. Devitrifying edges of glass shards, crystalline inclusions, and mineral grains that accompany glass in some tephras may show birefringence. Depending on chemical composition, volcanic glass may be colorless or relatively dark.
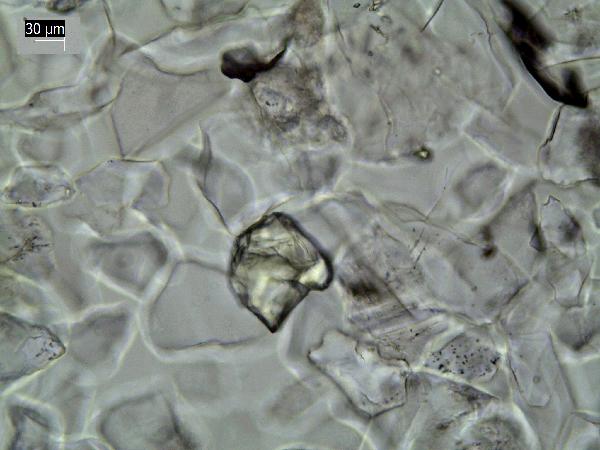
Pyrite, an iron sulfide, forms diagenetically in reducing environments with S and Fe. It appears as tiny (~1-10 μm) opaque euhedra and larger framboids, and filling in cavities in microfossils.
Vivianite, an iron phosphate, is the only common blue material in smear slides. (It is white and chalky on the core face when fresh, and oxidizes to bright blue over a few hours.) It forms diagenetically in reducing environments with P and Fe. It can indicate rapid burial and/or high P availability. Phosphorus is effectively recycled in the water column or at the sediment surface under normal conditions (it's frequently a limiting nutrient), but high sedimentation rates can sequester P in the sediments. Vivianite can form in association with the breakdown of P-rich materials such as animal and plant tissue (bones, seeds, etc.) and can be found as a coating in such objects as well as disseminated in the sediments.
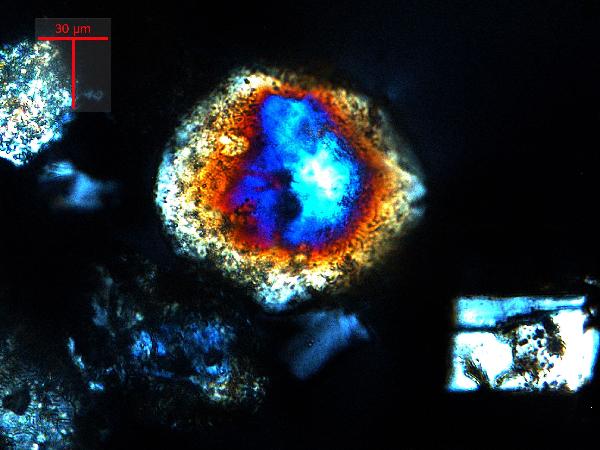
Iron oxides and hydroxides can be opaque or non-opaque, and span a range of mineralogies that are not always easily distinguished in smear slide. Opaque iron oxides, some of which are also classified as heavy minerals, require reflected light to aid in identification. Non-opaque iron (hydr)oxides include hematite and "limonite," which is actually a mixture of minerals including goethite.
Chitin is the material comprising the exoskeleton of insects, zooplankton, and other organisms. It appears in smear slides as transparent colorless to brown sheets in organic shapes (you can recognize body parts such as mandibles and antennae, or see how the curved edges of a plate would fit together with others). A polymer, it is readily distinguished from similarly-colored plant remains by its absence of internal cell structure.hematite (crystalline) in plane- and cross-polarized light
Phytoliths are intra- or extracellular amorphous silica deposits formed in the tissues of some plants. (Calcium oxalate phytoliths also exist.) They have many shapes, but are all isotropic and colorless (unless burned) and often have a purplish hue in plane light.
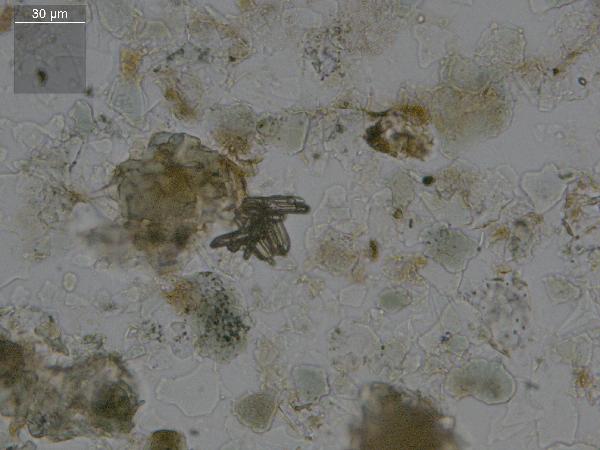
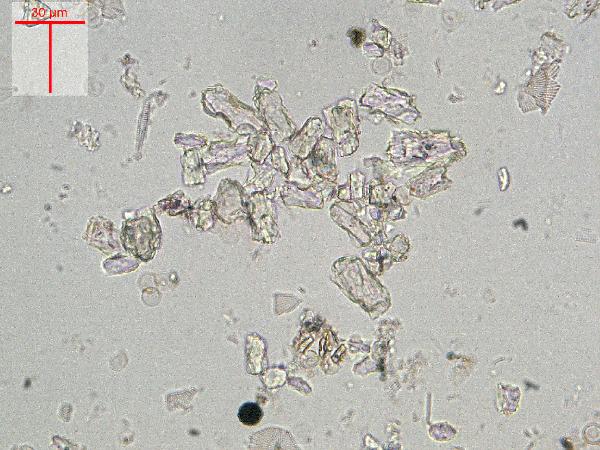
Sponge spicules are also composed of amorphous silica, and look somewhat like diatoms, but much larger - up to about 1000 μm. Double-ended needles are the most common freshwater form, and the groove running most of the length of the spicule is characteristic. Like phytoliths, they often appear purplish in plane light.
Volcanic glass is easy to identify in smear slides because it is isotropic. Glass shards may also have distinctive morphological features such as bubbles or broken bubble walls, stretched textures, and scythe-like edges, but isotropy is the diagnostic feature. Devitrifying edges of glass shards, crystalline inclusions, and mineral grains that accompany glass in some tephras may show birefringence. Depending on chemical composition, volcanic glass may be colorless or relatively dark.
 |
| volcanic glass in plane- and cross-polarized light |
Pyrite, an iron sulfide, forms diagenetically in reducing environments with S and Fe. It appears as tiny (~1-10 μm) opaque euhedra and larger framboids, and filling in cavities in microfossils.
 |
| pyrite euhedra |
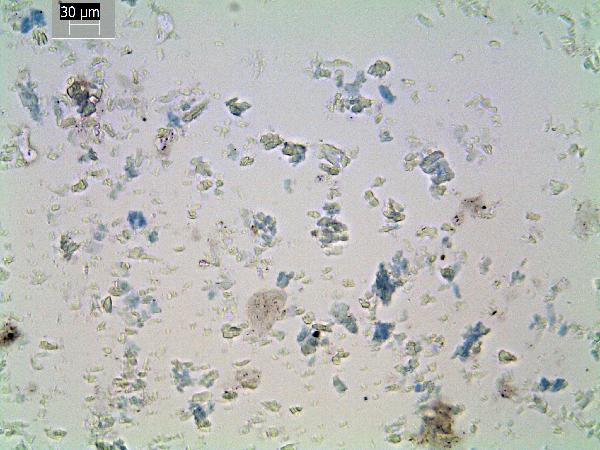 |
| vivianite (blue grains) |
| hematite (crystalline) in plane- and cross-polarized light |
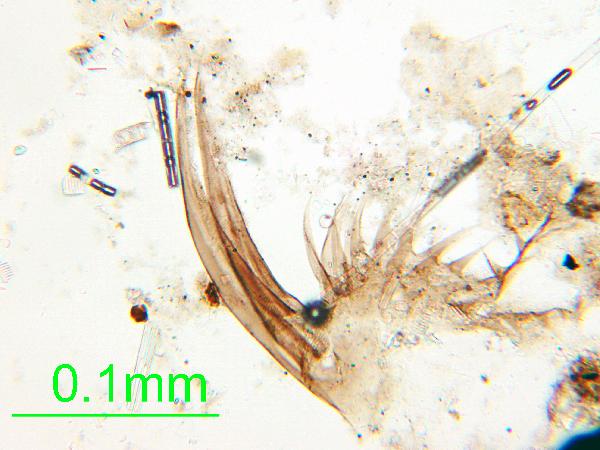 |
| zooplankton chitin |
Phytoliths are intra- or extracellular amorphous silica deposits formed in the tissues of some plants. (Calcium oxalate phytoliths also exist.) They have many shapes, but are all isotropic and colorless (unless burned) and often have a purplish hue in plane light.
 |
| one of the many shapes of phytoliths (purplish grain, center) |
Sponge spicules are also composed of amorphous silica, and look somewhat like diatoms, but much larger - up to about 1000 μm. Double-ended needles are the most common freshwater form, and the groove running most of the length of the spicule is characteristic. Like phytoliths, they often appear purplish in plane light.
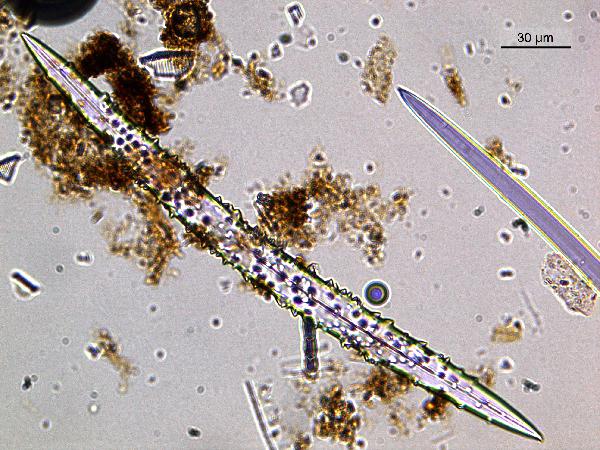 |
| sponge spicules, both bumpy and smooth |
TMI recommends that you keep track of your observations in a systematic way, such as by using one of our smear slide templates.



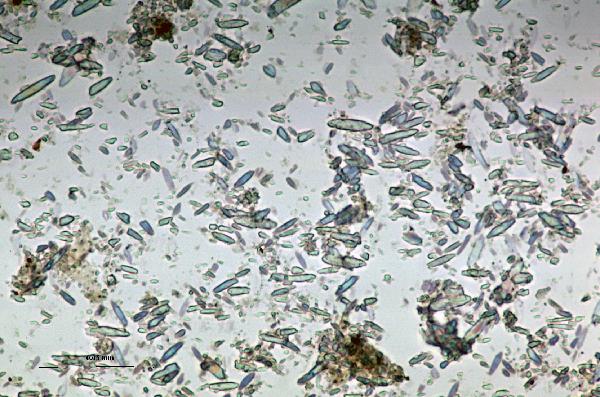
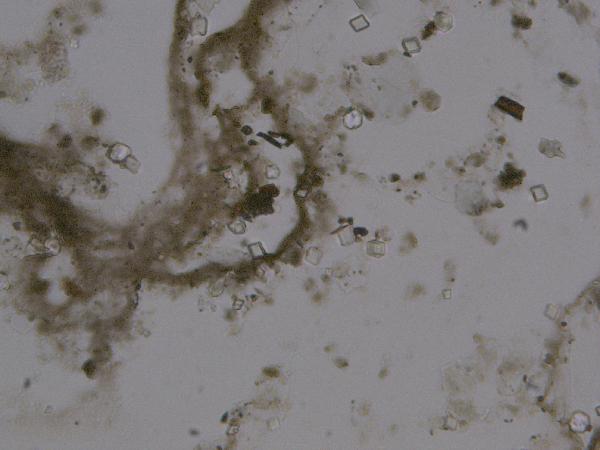

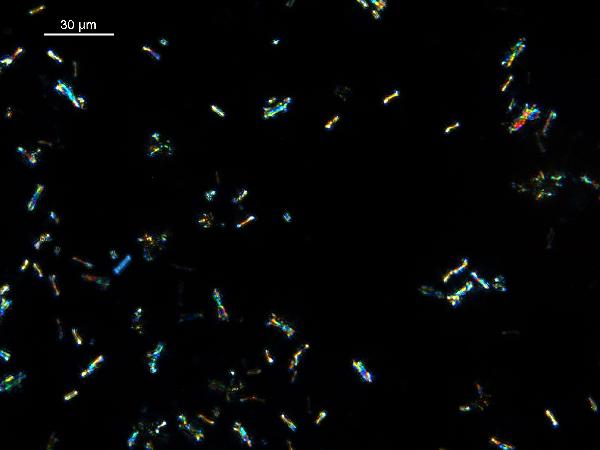
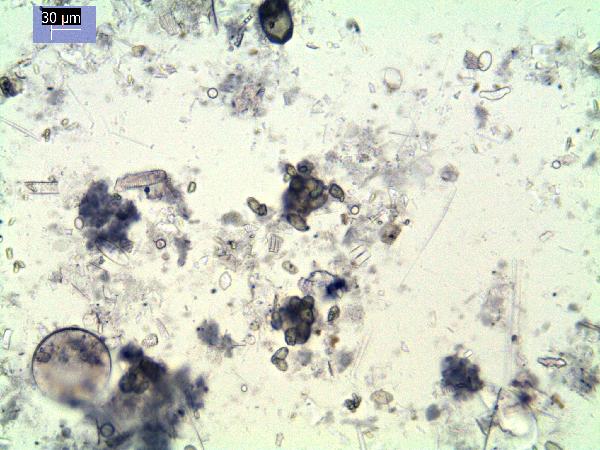
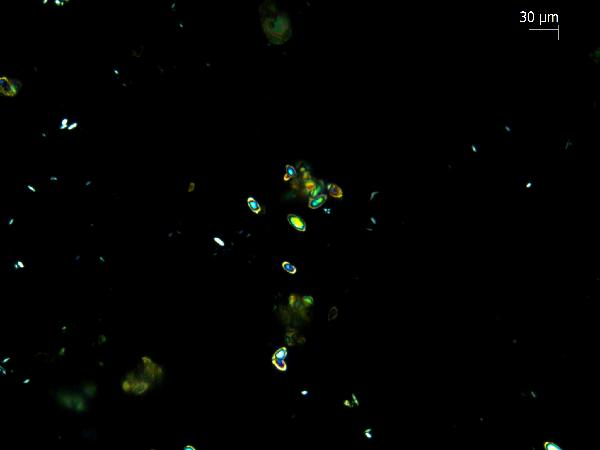

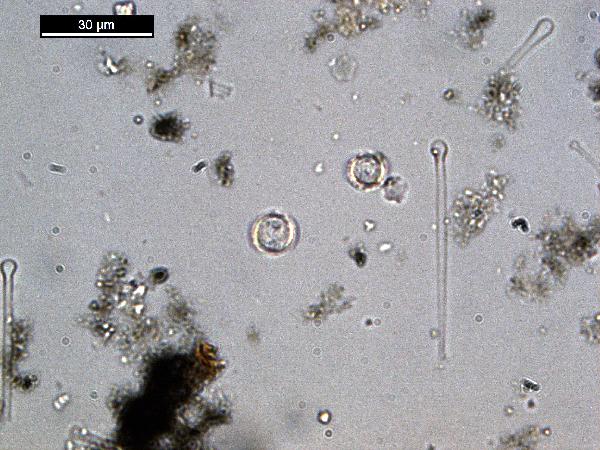
oxides,Non-opaque(Hematite/Limonite)/TAS-MACK11-1A-2B-1_5.5cm_hematite_pp-600.jpg)
oxides,Non-opaque(Hematite/Limonite)/TAS-MACK11-1A-2B-1_5.5cm_hematite_cp-600.jpg)
No comments:
Post a Comment